Health and Quality of Life
Our health has a major impact on our quality of life as humans — and it’s a no-brainer that most of us would prefer to stay as healthy as possible. To accomplish that, it helps to have recommendations backed by scientific evidence. This is where clinical research comes in.
Clinical research helps us understand what keeps humans healthy—and what factors may contribute to illness or disease. Findings from clinical research can help people make more informed decisions to protect their health and lead to safe, effective treatment or preventative measures when illness or injury arise.
Often, many clinical studies take place in urban areas where an organization carrying out a study is located. However, since our health is impacted by so many factors, including our local environments, it is important to allow rural Minnesotans a seat at the research table so that a study’s findings can benefit as many people as possible.
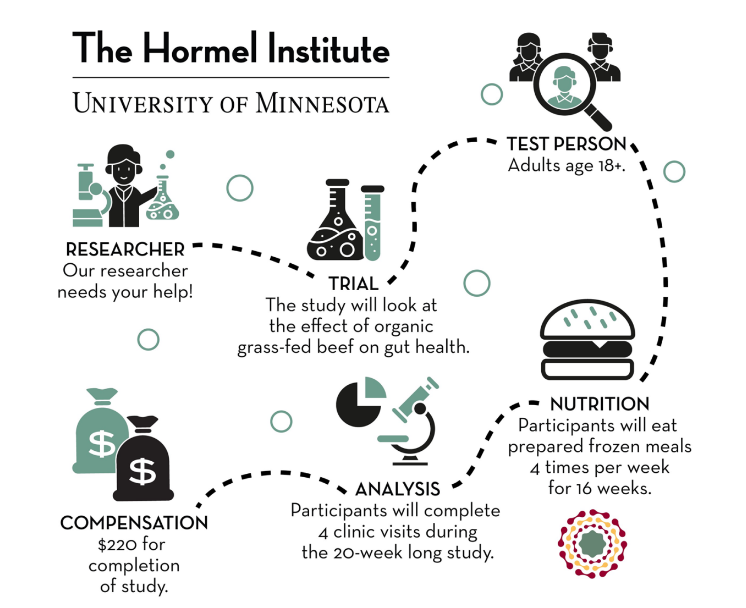
The Hormel Institute is proud to serve as a site for various clinical research studies that help represent the individuals who make up our rural communities in southeastern Minnesota.
Participants Still Needed for 10,000 Families Study
The Hormel Institute is excited to announce our partnership with the 10,000 Families Study (10KFS) of the University of Minnesota! 10KFS is a study of family health across Minnesota, looking at how environment, genetics, and daily life affect health and illness over time. We are partnering to invite families from Mower County and broader Southeast Minnesota to join the study; let’s make sure our region is represented in this landmark project! We’re also offering the opportunity to come over to the Institute for health visits, the in-person part of 10KFS, rather than go up to the Twin Cities.
Want to see if your family is eligible to join 10KFS? Click on z.umn.edu/10KFS-Hormel.
Questions? Email tenkfs@umn.edu.
For more information, contact:
Emily Heath, RN BSN
Clinical Research and Outreach Nurse
The Hormel Institute University of Minnesota
Office: 507-437-9642
Email: eheath@umn.edu
FAQS
Clinical trials vs. clinical studies: What’s the difference?
Clinical trials and clinical studies are both terms that can come up in conversations about clinical research. While they might seem similar on the surface, they do have some important distinctions.
Clinical trials involve some sort of intervention—such as the use of medicine or surgery—to study its safety and effectiveness.
Clinical studies is a broader term that encompasses clinical trials.
There are different types of clinical studies: some may involve an intervention (i.e., a clinical trial), while others will not involve an intervention and instead monitor people and their health in an unaltered setting (an observational study).